 |
 |
|
|
 |
||
| Annual Report | ||
 |
News | |
| MDRC Administration | ||
 |
Directory / Bottin | |
 |
Contact Us | |
 |
Directions / Maps | |
 |
Steering Committee | |
| Research | ||
 |
Events & Seminars | |
 |
MDRC at the forefront | |
 |
McGarry Lecture | |
 |
Cahill Lecture | |
 |
MDRC Core Facilities | |
 |
Scientific Links | |
 |
Education & Employment | |
| Funding | ||
 |
MDRC Grants | |
 |
Donations | |
 |
Non-Profit & Industrial Partners | |
| Medical Information | ||
 |
JDRF | |
 |
CDA | |
 |
Diabetes Quebec | |
 |
ADA | |
 |
IDF | |
 |
NIH / NIDDK | |
 |
Alfediam | |
 |
EASD | |
| Home / About Us > Dr Marc Prentki | 
Contact info Dr Marc Prentki Tel: 1-514-890-8000, ext. 23642
Research keywords
|
|
Marc Prentki, PhD
Professor of Nutrition and Biochemistry
Canada Research Chair in Diabetes and Metabolism MDRC Director Biographical Sketch
Marc Prentki was trained in Biochemistry at the University of Geneva where he obtained a PhD degree in 1980. His thesis was in the field of the cytoskeleton, under the direction of Dr B. Jeanrenaud. He then joined the laboratory of Prof. Albert Renold in Geneva, where he worked as a post-doctoral fellow on intracellular Ca2+ homeostasis and insulin secretion. He next joined in 1984 the laboratory of Dr F. Matschinsky at the University of Pennsylvania to work as a postdoctoral fellow on Ca2+ signaling, and was soon promoted to Research Assistant Professor in the Department of Biochemistry and Biophysics. He returned to Geneva in 1987 to work as an Assistant Professor at the Institut de Biochimie Clinique. His research focus was on pancreatic β-cell metabolic signaling and glucose regulation of gene expression. In 1994 he joined the Departments of Nutrition and Biochemistry at the Universit� de Montr�al where he is now full Professor. In 2004, Dr Prentki, with the help of several investigators, founded the Montreal Diabetes Research Center that he currently directs. He was awarded in 1994 the prize of the Federations of European Endocrine Societies, a Canada Research Chair in Diabetes and Metabolism in 2006 and was awarded the Albert Renold prize of the European Association for the Study of Diabetes in 2011. Selected Scientific Contributions
Marc Prentki showed in the 1980s that inositol 1,4,5-trisphosphate is a second messenger causing early mobilization of Ca2+ from the endoplasmic reticulum. He also observed using microfluorimetry that Ca2+ signals are oscillatory in the β-cell in response to carbamylcholine and that the frequency of oscillations vary as a function of the agonist concentration. This suggested that frequency in addition to amplitude encodes cellular Ca2+ signaling. The research of Marc Prentki has increased our understanding of the mechanism of glucose and fatty acid regulated insulin secretion in health, obesity and diabetes. Notably he proposed together with Dr B. Corkey that malonyl-CoA acts as a metabolic signaling molecule in the β-cell and that anaplerosis is implicated in glucose-induced insulin secretion. Collaborative work with other groups has shown that malonyl-CoA also regulates insulin action, food intake and body weight at the level of the hypothalamus. In 1996, he introduced together with B. Corkey the concept of glucolipotoxicity that has important implication for the development of type 2 diabetes. His group also first showed that GLP1 promotes β-cell growth and protects the β-cell from the glucolipotoxic insult. Recent work from his lab led to the concept that glycerolipid/fatty acid (GL/FA) cycling acts as a signaling and fuel detoxification machine producing signals for insulin secretion and action as well as cell growth. In particular long chain saturated monoacylglycerol was identified as a metabolic coupling factor for glucose-induced insulin secretion. With respect to the field of metabolism and cancer, he provided possible links between obesity and cancer by showing that unsaturated fatty acids activate PI3-Kinase and the protooncogene PKB/Akt in breast cancer cells, and that their action on cell growth involves the fatty acid receptor GPR40 and possibly enhanced GL/FA cycling. Finally he co-directed with Drs B. Posner, R. Sladek and P. Froguel a large-scale project on the genetics of type 2 diabetes. It led to a landmark publication in Nature (2007), the first successful whole genome scan for any disease and the identification of novel susceptibility loci. Research Interests
Current projects in the laboratory fall into four areas. The laboratory addresses these investigations using a broad panoply of technologies, including rodent KO models, RNA silencing, metabolomics/proteomics and transcriptomics, in vivo assessment of glucose/lipid metabolism and insulin signaling, as well as biochemical assays. The group works on both basic research and industry funded projects related to drug and biomarkers discovery. Metabolic signal transduction in the pancreatic β-cell. We seek to identify the signals that mediate the link between fuel metabolism in the β-cell and insulin release. In this respect a particular effort is made on the study of several enzymes and metabolites of the GL/FA cycling. We also address the question of the pathways that negatively inpinge on insulin secretion, particularly via “metabolic deceleration”. 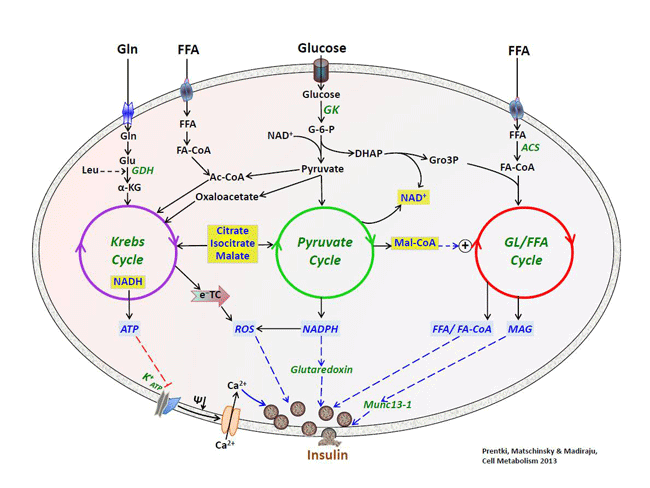 Relationship between three fuel-driven metabolic cycles that generate metabolic coupling
factors in the β-cell Molecular basis of β-cell compensation and failure in type 2 diabetes. Various animal models of obesity and type 2 diabetes are used to define the relative contributions of altered β-cell growth and apoptosis, exhaustion and metabolic signal transduction. With respect to β-cell adaptation to fuel surfeit and glucolipotoxicity the focus of the research is on the role of GL/FA cycling and the unbiased discovery of novel patways. Fuel detoxification processes. Very little is known about the processes whereby cells detoxify the excess of nutrients they face in obesity and diabetes. Using metabolomics and candidate genes aproaches our group is currently identifying novel pathways and enzymes implicated in this process in the endocrine pancreas and other tissues. Novel targets for obesity and metabolic syndrome. We study enzymes and pathways of the GL/FA cycle implicated in the control of energy homeostasis, insulin sensitivity, appetite and adipose tissue browning. In collaboration with other teams at the MDRC we investigate the role of hypothalamic and brain reward areas in these processes. 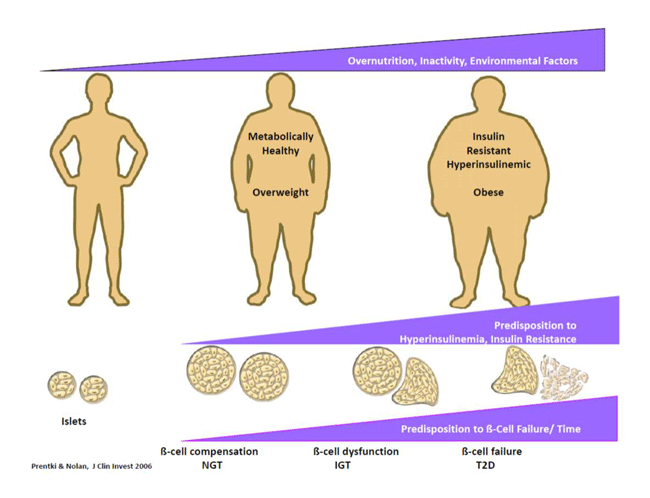 Islet β-cell failure and the natural history of T2D

Prentki laboratory members, February 2014 |
|
| � Montreal Diabetes Research Center 2018 |
| Home / About Us News Directory / Bottin Contact Us |