 |
 |
|
|
 |
||
| Annual Report | ||
 |
News | |
| MDRC Administration | ||
 |
Directory / Bottin | |
 |
Contact Us | |
 |
Directions / Maps | |
 |
Steering Committee | |
| Research | ||
 |
Events & Seminars | |
 |
MDRC at the forefront | |
 |
McGarry Lecture | |
 |
Cahill Lecture | |
 |
MDRC Core Facilities | |
 |
Scientific Links | |
 |
Education & Employment | |
| Funding | ||
 |
MDRC Grants | |
 |
Donations | |
 |
Non-Profit & Industrial Partners | |
| Medical Information | ||
 |
JDRF | |
 |
CDA | |
 |
Diabetes Quebec | |
 |
ADA | |
 |
IDF | |
 |
NIH / NIDDK | |
 |
Alfediam | |
 |
EASD | |
| Home / About Us > Dr Corinne Hoesli | 
Contact info Dr Corinne Hoesli Tel: 1-514-398-4275
Research keywords
|
|
Corinne Hoesli, ing. jr, PhD
Assistant Professor in Chemical Engineering
Biographical Sketch
Dr Corinne Hoesli is an Assistant Professor in Chemical Engineering at McGill University, Montreal, QC, Canada. She is a biochemical engineer with expertise in bioprocess development, high throughput screening and stem cell culture optimization applied to diabetes cellular therapy. She obtained undergraduate degrees in Biochemistry and Chemical Engineering in 2003 at the University of Ottawa. She then moved to Vancouver to complete a PhD in Chemical and Biological engineering at the University of British Columbia in the laboratory of Dr James Piret. Her doctoral work focused on bioprocess development for the cell-based treatment of diabetes. She next pursued postdoctoral training in the bioengineering laboratories of Dr Alain Garnier and Dr Ga�tan Laroche, where she designed a live cell imaging system to study the effect of biomimetic molecules on vascular regeneration. She became the head of the Stem Cell Bioprocessing Laboratory at McGill in August 2014. Her laboratory seeks to combine pancreatic cell encapsulation approaches and vascular biomaterials to overcome the limitations of islet transplantation to treat diabetes. She has received numerous awards recognizing her leadership in cell culture engineering, including the Martin Sinacore Outstanding Young Investigator Award conferred by Engineering Conferences International and Biogen Idec. Selected Scientific Contributions
Research Interests
Islet transplantation has emerged as a novel long-term treatment for type 1 diabetes, avoiding daily insulin administration for approximately 3 years in most patients. The access to this therapeutic option is limited by the amount of islet supply from donors, as well as the requirement for lifelong immunosuppression to avoid graft rejection. To overcome these limitations, alternative cell sources and methods to minimize graft rejection are needed. Islet encapsulation relies on creating a protective barrier around the islets to reduce the access of the immune system to the graft. Preclinical trials in primates and some trials of encapsulated islet transplantation in humans have highlighted the lack of consistency in the efficacy of encapsulated islets, even within the same research group. Professor Hoesli's research interest lie in understanding and overcoming the challenges of therapeutic cell production to treat diabetes. Optimizing islet encapsulation methods. Bioprocess engineering is a discipline that focuses on developing systematic and optimized methods to handle cells and generate biological products with high consistency. Professor Hoesli applies these engineering principles to develop more robust and scalable methods to produce and encapsulate therapeutic cells to treat diabetes. She has developed an emulsion-based method to encapsulate large amounts of islets in a single batch in less than 30 min, compared to several hours required with typical encapsulation methods. Moreover, this method can produce hydrogel beads that are less permeable to harmful antibodies and less immunogenic. This method could improve the outcome of encapsulated islet transplantation. Producing islets from stem cells. The broad implementation of islet encapsulation to treat diabetes would require a 100-fold increased availability of donor cells. Instead, islets could be produced at clinical scale using pluripotent stem cells. Induced pluripotent stem cells can be obtained from most cells in the human body. For example, the fibroblasts found in a simple skin biopsy can be reprogrammed into an embryonic stem cell-like state. These reprogrammed cells can then be differentiated into pancreatic cells, including insulin-producing cells. Even with recent improvements in differentiation protocols, the yields of insulin-producing cells remain low. Professor Hoesli is investigating the effect of different biomaterials on the yield of insulin-producing cells from induced pluripotent stem cells. Engineering an artificial pancreas. The lack of oxygen supply after encapsulated islet transplantation is a major source of islet cell losses. To overcome this issue, the Hoesli lab is developing a device that would supply nutrients including oxygen to the cells after transplantation. The short-term goal of this project is to develop and test the device in the laboratory, where it could serve as an in vitro model to better understand pancreas cell biology. The long-term goal of this project is to develop a device that could be transplanted to treat diabetes. 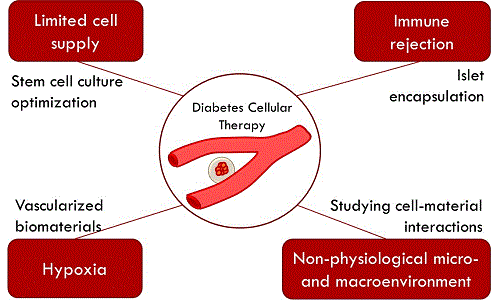
|
|
| � Montreal Diabetes Research Center 2018 |
| Home / About Us News Directory / Bottin Contact Us |