 |
 |
|
|
 |
||
| Annual Report | ||
 |
News | |
| MDRC Administration | ||
 |
Directory / Bottin | |
 |
Contact Us | |
 |
Directions / Maps | |
 |
Steering Committee | |
| Research | ||
 |
Events & Seminars | |
 |
MDRC at the forefront | |
 |
McGarry Lecture | |
 |
Cahill Lecture | |
 |
MDRC Core Facilities | |
 |
Scientific Links | |
 |
Education & Employment | |
| Funding | ||
 |
MDRC Grants | |
 |
Donations | |
 |
Non-Profit & Industrial Partners | |
| Medical Information | ||
 |
JDRF | |
 |
CDA | |
 |
Diabetes Quebec | |
 |
ADA | |
 |
IDF | |
 |
NIH / NIDDK | |
 |
Alfediam | |
 |
EASD | |
| Home / About Us > Dr Barry Posner | Contact info Dr Barry I. Posner Tel: 1-514-934-1934
Research keywords
|
|
Barry Posner, MD
Professor of Medicine and Anatomy & Cell Biology
MDRC Co-Director/McGill Biographical Sketch
Dr Barry I. Posner is a Professor in the Departments of Medicine, Anatomy & Cell Biology, and Director of the Polypeptide and Protein Hormone Laboratory at McGill University. A gold medalist in his graduating class, Posner pursued post-graduate work at the Massachusetts Institute of Technology, and the National Institutes of Health in Bethesda, MD before joining the Royal Victoria Hospital and the McGill University Faculty of Medicine in 1970. In 1979 he became full professor at McGill and senior physician at the Royal Victoria Hospital. He has served as Director of the McGill Endocrine training program and physician-in-chief at the Sir Mortimer B. Davis Jewish General Hospital. His fundamental research on insulin signaling led to the discovery of the endosomal system and the view that this is a central site for both initiating and regulating signal transduction. Posner's current diabetes research focuses on the genes responsible for diabetes, the discovery of which will aid in the prediction and treatment of this disease. He has published over 275 scientific manuscripts, and, as a Visiting Professor, has delivered numerous prestigious lectures including the Banting and Best Memorial Lecture of the International Diabetes Federation (1991), the Pfizer Lectures at Harvard University (1993), the Joe Doupe Memorial Lecture at the University of Manitoba (1994), the Novartis lecture of the Canadian Society of Endocrinology and Metabolism (1997), the David M. Kovitz Memorial Lecture at the University of Calgary (2000), the 2nd John & Mary Davidson Lecture (and Award) of the University of Toronto in 2002, and the Distinguished Service Award of the Canadian Society of Endocrinology and Metabolism (2008). His academic contributions have been recognized by election to the Association of American Physicians (1988), receipt of the Distinguished Scientist award of the CSCI (1991), election to Fellowship in the Royal Society of Canada, appointment as Officer of the Order of Canada (1999), and receipt of the Queen's Golden Jubilee Medal (2002). Selected Scientific Contributions
We discovered and/or defined the following: New roles for peptide hormone receptors. Observed in tissues not previously regarded as targets (e.g. IRs in placenta) [1972 - 74]. Identified in intracellular vesicular elements suggesting intracellular signaling [1975 - 78]. Discovered peptide hormones up-regulate own receptors [1974, 1988]. Pioneered use of EM radioautography to identify new hormone targets and was the first to show receptors in the circumventricular organs of CNS and the endothelium of microvessels [1977 - 79]. Role of endosomes in cell signaling: Demonstrated internalization of hormones into unique intracellular elements [1979-82] and proposed concept of Signaling Endosome[1980]. Demonstrated signaling by insulin and EGF in endosomes [1986-94]. Endosomes as key site of IRK regulation: Selective insulin degradation by an endosomal acidic insulinase (EAI) [1988-94]. Dephosphorylation and inactivation IRK by associated phosphotyrosine phosphatase(s) (PTP) [1992-98]. Peroxovanadium compounds activate IRK in endosomes and yield full insulin response [1988-1996]. Therefore activation of the IRK is not only necessary but is sufficient for insulin action. Acidification of endosomes changes conformation and inactivates IRK [1998]. Demonstrated PTP1B is not IRK-associated PTP in endosomes [2006]. Cloning of new signaling molecules: First to describe IRS-3 [1996]. Cloned 1st PTP with SH2 domain (PTP1C or SHPTP1) [1991]. Cloned rat Gab2 - a key docking protein for EGFR [1999]. EGF action: Showed key role for PI-3K in mitogenesis [1999], via the phosphorylation of Gab2 [2000]. Proved Gab2 is the key docking molecule for Grb2, SHPTP2 and Src (constitutive)[2000-03], and that Src activation needed for Gab2 tyrosine phosphorylation and mitogenesis [2003]. Demonstrated that EGF action involves vATPase-dependent activation of mTOR [2009]. Insulin and IGF signaling: IGF feeds-back directly on the pituitary to inhibit growth hormone secretion [1983]. Insulin suppresses IGFBP-1 transcription via the activation of mTOR [1999]. The phosphorylation of FOXO transcription factors is not required [2006]. Signaling in rafts: Insulin signaling in hepatocytes requires intact rafts [2004]. EGF activates EGFR in and recruits signaling molecules to cell surface and endosomal rafts including those in late endosomes. Caveolin is not involved in either the concentration nor internalization of signaling rafts [2007-2009]. New genes in type 2 diabetes mellitus (T2DM): PI on Genome Canada/Quebec project which discovered new genetic loci responsible for T2DM [2007 & 2009]. 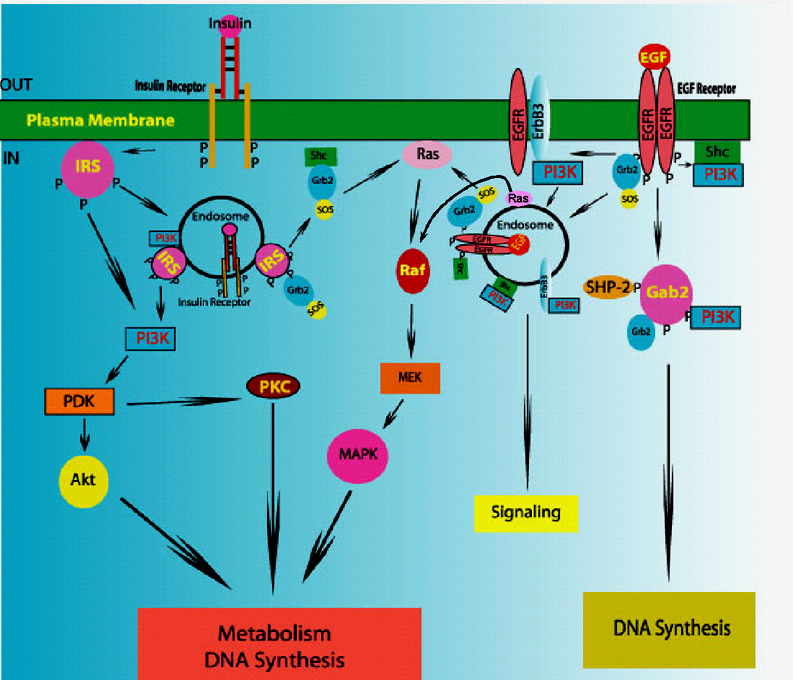 Research Interests
My laboratory focuses on the study of insulin and growth factor action with a particular emphasis on their relationship to the pathogenesis of type 2 diabetes mellitus. Both insulin and IGF bind to their cell surface receptors, which are tyrosine kinases, leading to their activation and internalization into the endosomal system. Novel substrates of both the insulin receptor kinase (IRK) and the EGF receptor kinase (EGFRK) have been identified within endosomes. We identified a novel class of phosphotyrosine phosphatase (PTP) inhibitors - the peroxovanadium (pV) compounds - as potent insulin mimickers. pVs promote activation of the IRK, both in situ and in vivo by inhibiting an IRK-associated PTP, leading to IRK activation and a range of insulin effects, including hypoglycemia in normal and diabetic rats. A current focus is on the role of intraendosomal processes as regulators of IRK function. These include the modulation of intraendosomal pH, a specific endosomal acidic insulinase (EAI), and PTPs, all of which modulate IRK activation and insulin signaling. Defects in one or more of these processes could contribute to the pathogenesis of T2DM. We are following up these studies to further our understanding of insulin and growth factor signaling and their abnormalities. In this regard emphasis is being placed on how EGF and insulin signaling use very similar intracellular pathways and yet produce responses highly specific to each agent. |
|
| � Montreal Diabetes Research Center 2018 |
| Home / About Us News Directory / Bottin Contact Us |